FiercePharmaAsia—China's new drug regulator, Teva's Celltrion headache, NextCODE-Google tie-up | Fierce Pharma

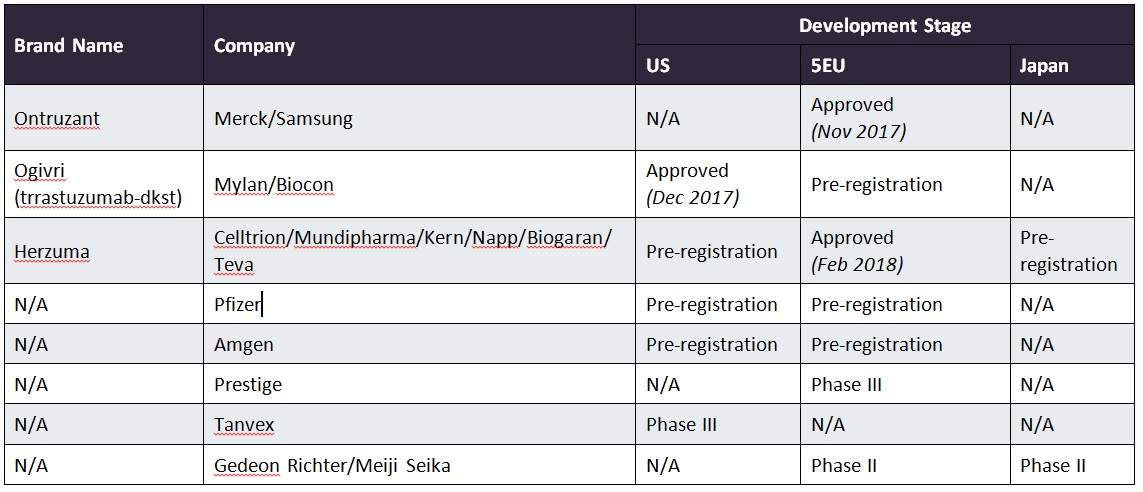
Teva two: FDA approves Celltrion-made Herceptin biosimilar - BioProcess InternationalBioProcess International

Teva and Celltrion Launch Truxima (biosimilar- rituximab) to Treat Wegener's Granulomatosis and Microscopic Polyangiitis in the US